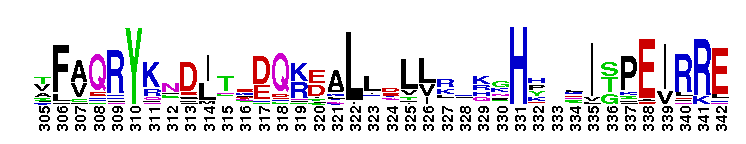
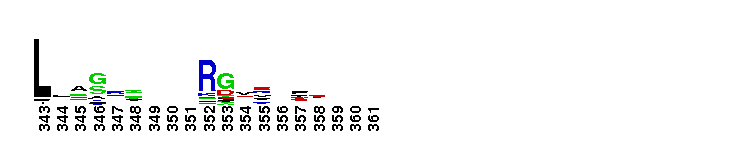
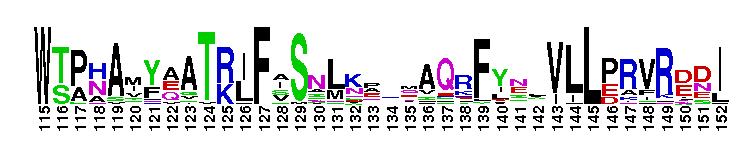| |||||||||||||||||||
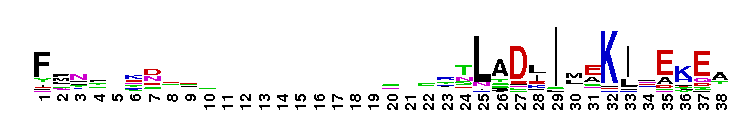
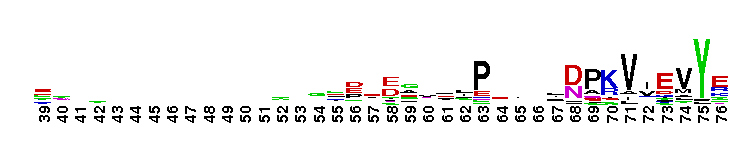
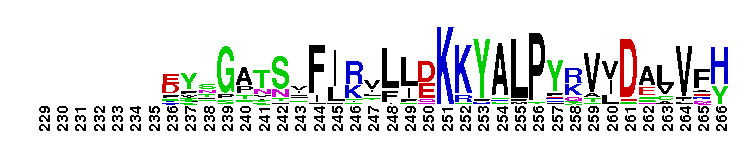
Tips:  Range on the Protein: Protein ID Protein Position Domain Position:  No Conserved Features/Sites Found for Bystin No Conserved Features/Sites Found for Bystin
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 Bystin. Trophinin and tastin form a cell adhesion molecule complex that potentially mediates an initial attachment of the blastocyst to uterine epithelial cells at the time of implantation. Trophinin and tastin bind to an intermediary cytoplasmic protein called bystin. Bystin may be involved in implantation and trophoblast invasion because bystin is found with trophinin and tastin in the cells at human implantation sites and also in the intermediate trophoblasts at invasion front in the placenta from early pregnancy. This family also includes the yeast protein ENP1. ENP1 is an essential protein in Saccharomyces cerevisiae and is localised in the nucleus. It is thought that ENP1 plays a direct role in the early steps of rRNA processing as enp1 defective yeast cannot synthesise 20S pre-rRNA and hence 18S rRNA, which leads to reduced formation of 40S ribosomal subunits.
Bystin. Trophinin and tastin form a cell adhesion molecule complex that potentially mediates an initial attachment of the blastocyst to uterine epithelial cells at the time of implantation. Trophinin and tastin bind to an intermediary cytoplasmic protein called bystin. Bystin may be involved in implantation and trophoblast invasion because bystin is found with trophinin and tastin in the cells at human implantation sites and also in the intermediate trophoblasts at invasion front in the placenta from early pregnancy. This family also includes the yeast protein ENP1. ENP1 is an essential protein in Saccharomyces cerevisiae and is localised in the nucleus. It is thought that ENP1 plays a direct role in the early steps of rRNA processing as enp1 defective yeast cannot synthesise 20S pre-rRNA and hence 18S rRNA, which leads to reduced formation of 40S ribosomal subunits. No pairwise interactions found for the domain Bystin
No pairwise interactions found for the domain Bystin