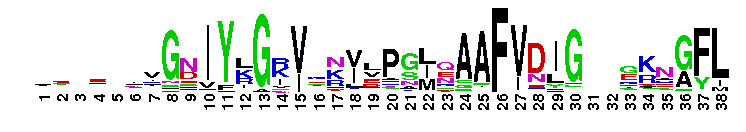| |||||||||||||||||||
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 S1_RNase_E: RNase E and RNase G, S1-like RNA-binding domain. RNase E is an essential endoribonuclease in the processing and degradation of RNA. In addition to its role in mRNA degradation, RNase E has also been implicated in the processing of rRNA, and the maturation of tRNA, 10Sa RNA and the M1 precursor of RNase P. RNase E associates with PNPase (3' to 5' exonuclease), Rhl B (DEAD-box RNA helicase) and enolase (glycolytic enzyme) to form the RNA degradosome. RNase E tends to cut mRNA within single-stranded regions that are rich in A/U nucleotides. The N-terminal region of RNase E contains the catalytic site. Within the conserved N-terminal domain of RNAse E and RNase G, there is an S1-like subdomain, which is an ancient single-stranded RNA-binding domain. S1 domain is an RNA-binding module originally identified in the ribosomal protein S1. The S1 domain is required for RNA cleavage by RNase E. RNase G is paralogous to RNase E with an N-terminal catalytic domain that is highly homologous to that of RNase E. RNase G not only shares sequence similarity with RNase E, but also functionally overlaps with RNase E. In Escherichia coli, RNase G is involved in the maturation of the 5' end of the 16S rRNA. RNase G plays a secondary role in mRNA decay.
S1_RNase_E: RNase E and RNase G, S1-like RNA-binding domain. RNase E is an essential endoribonuclease in the processing and degradation of RNA. In addition to its role in mRNA degradation, RNase E has also been implicated in the processing of rRNA, and the maturation of tRNA, 10Sa RNA and the M1 precursor of RNase P. RNase E associates with PNPase (3' to 5' exonuclease), Rhl B (DEAD-box RNA helicase) and enolase (glycolytic enzyme) to form the RNA degradosome. RNase E tends to cut mRNA within single-stranded regions that are rich in A/U nucleotides. The N-terminal region of RNase E contains the catalytic site. Within the conserved N-terminal domain of RNAse E and RNase G, there is an S1-like subdomain, which is an ancient single-stranded RNA-binding domain. S1 domain is an RNA-binding module originally identified in the ribosomal protein S1. The S1 domain is required for RNA cleavage by RNase E. RNase G is paralogous to RNase E with an N-terminal catalytic domain that is highly homologous to that of RNase E. RNase G not only shares sequence similarity with RNase E, but also functionally overlaps with RNase E. In Escherichia coli, RNase G is involved in the maturation of the 5' end of the 16S rRNA. RNase G plays a secondary role in mRNA decay. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.