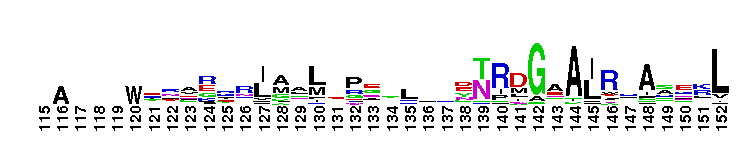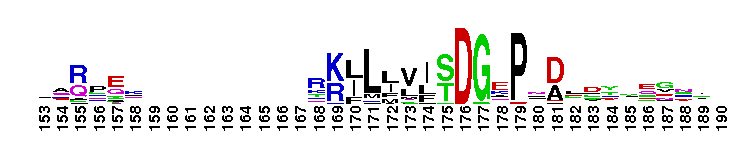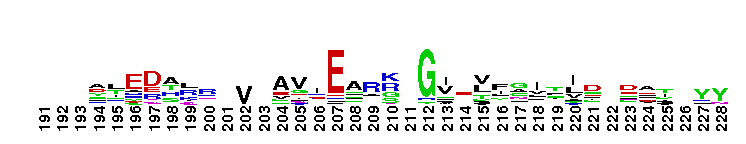| ||||||||||||||||||||||||||||
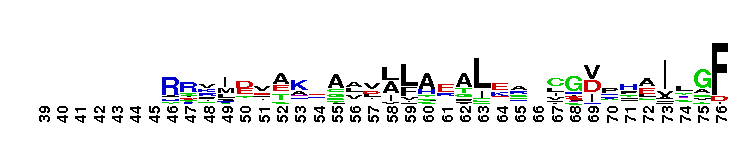
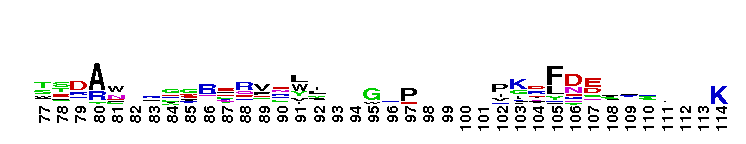
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 norD type: Denitrifying bacteria contain both membrane bound and periplasmic nitrate reductases. Denitrification plays a major role in completing the nitrogen cycle by converting nitrate or nitrite to nitrogen gas. The pathway for microbial denitrification has been established as NO3- ------> NO2- ------> NO -------> N2O ---------> N2. This reaction generally occurs under oxygen limiting conditions. Genetic and biochemical studies have shown that the first srep of the biochemical pathway is catalyzed by periplasmic nitrate reductases. This family is widely present in proteobacteria and firmicutes. This version of the domain is also present in some archaeal members. The function of the vWA domain in this sub-group is not known. Members of this subgroup have a conserved MIDAS motif.
norD type: Denitrifying bacteria contain both membrane bound and periplasmic nitrate reductases. Denitrification plays a major role in completing the nitrogen cycle by converting nitrate or nitrite to nitrogen gas. The pathway for microbial denitrification has been established as NO3- ------> NO2- ------> NO -------> N2O ---------> N2. This reaction generally occurs under oxygen limiting conditions. Genetic and biochemical studies have shown that the first srep of the biochemical pathway is catalyzed by periplasmic nitrate reductases. This family is widely present in proteobacteria and firmicutes. This version of the domain is also present in some archaeal members. The function of the vWA domain in this sub-group is not known. Members of this subgroup have a conserved MIDAS motif. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.