| ||||||||||||||||||||||
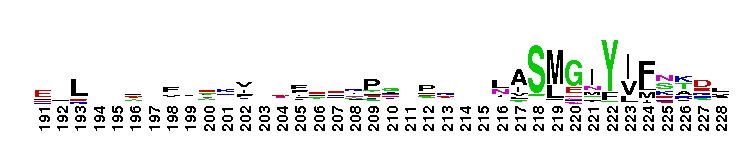
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 ADP-glucose pyrophosphorylase is involved in the biosynthesis of glycogen or starch. ADP-glucose pyrophosphorylase (glucose-1-phosphate adenylyltransferase) catalyzes a very important step in the biosynthesis of alpha 1,4-glucans (glycogen or starch) in bacteria and plants: synthesis of the activated glucosyl donor, ADP-glucose, from glucose-1-phosphate and ATP. ADP-glucose pyrophosphorylase is a tetrameric allosterically regulated enzyme. While a homotetramer in bacteria, in plant chloroplasts and amyloplasts, it is a heterotetramer of two different, yet evolutionary related, subunits. There are a number of conserved regions in the sequence of bacterial and plant ADP-glucose pyrophosphorylase subunits. It is a subfamily of a very diverse glycosy transferase family 2.
ADP-glucose pyrophosphorylase is involved in the biosynthesis of glycogen or starch. ADP-glucose pyrophosphorylase (glucose-1-phosphate adenylyltransferase) catalyzes a very important step in the biosynthesis of alpha 1,4-glucans (glycogen or starch) in bacteria and plants: synthesis of the activated glucosyl donor, ADP-glucose, from glucose-1-phosphate and ATP. ADP-glucose pyrophosphorylase is a tetrameric allosterically regulated enzyme. While a homotetramer in bacteria, in plant chloroplasts and amyloplasts, it is a heterotetramer of two different, yet evolutionary related, subunits. There are a number of conserved regions in the sequence of bacterial and plant ADP-glucose pyrophosphorylase subunits. It is a subfamily of a very diverse glycosy transferase family 2. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.