| |||||||||||||||||||
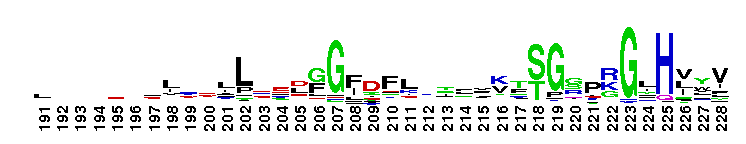
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 AE_Prim_S_like: primase domain similar to that found in the small subunit of archaeal and eukaryotic (A/E) DNA primases. The replication machineries of A/Es are distinct from that of bacteria. Primases are DNA-dependent RNA polymerases which synthesis the short RNA primers required for DNA replication. In eukaryotes, this small catalytically active primase subunit (p50) and a larger primase subunit (p60), referred to jointly as the core primase, associate with the B subunit and the DNA polymerase alpha subunit in a complex, called Pol alpha-pri. In addition to its catalytic role in replication, eukaryotic DNA primase may play a role in coupling replication to DNA damage repair and in checkpoint control during S phase. Pfu41 and Pfu46 comprise the primase complex of the archaea Pyrococcus furiosus; these proteins have sequence identity to the eukaryotic p50 and p60 primase proteins respectively. Pfu41 preferentially uses dNTPs as substrate. Pfu46 regulates the primase activity of Pfu41. Also found in this group is the primase-polymerase (primpol) domain of replicases from archaeal plasmids including the ORF904 protein of pRN1 from Sulfolobus islandicus (pRN1 primpol). The pRN1 primpol domain exhibits DNA polymerase and primase activities; a cluster of active site residues (three acidic residues, and a histidine) is required for both these activities. The pRN1 primpol primase activity prefers dNTPs to rNTPs; however incorporation of dNTPs requires rNTP as cofactor. This group also includes the Pol domain of bacterial LigD proteins such Mycobacterium tuberculosis (Mt)LigD. MtLigD contains an N-terminal Pol domain, a central phosphoesterase module, and a C-terminal ligase domain. LigD Pol plays a role in non-homologous end joining (NHEJ)-mediated repair of DNA double-strand breaks (DSB) in vivo, perhaps by filling in short 5'-overhangs with ribonucleotides; the filled in termini would be sealed by the associated LigD ligase domain. The MtLigD Pol domain is stimulated by manganese, is error-prone, and prefers adding rNTPs to dNTPs in vitro.
AE_Prim_S_like: primase domain similar to that found in the small subunit of archaeal and eukaryotic (A/E) DNA primases. The replication machineries of A/Es are distinct from that of bacteria. Primases are DNA-dependent RNA polymerases which synthesis the short RNA primers required for DNA replication. In eukaryotes, this small catalytically active primase subunit (p50) and a larger primase subunit (p60), referred to jointly as the core primase, associate with the B subunit and the DNA polymerase alpha subunit in a complex, called Pol alpha-pri. In addition to its catalytic role in replication, eukaryotic DNA primase may play a role in coupling replication to DNA damage repair and in checkpoint control during S phase. Pfu41 and Pfu46 comprise the primase complex of the archaea Pyrococcus furiosus; these proteins have sequence identity to the eukaryotic p50 and p60 primase proteins respectively. Pfu41 preferentially uses dNTPs as substrate. Pfu46 regulates the primase activity of Pfu41. Also found in this group is the primase-polymerase (primpol) domain of replicases from archaeal plasmids including the ORF904 protein of pRN1 from Sulfolobus islandicus (pRN1 primpol). The pRN1 primpol domain exhibits DNA polymerase and primase activities; a cluster of active site residues (three acidic residues, and a histidine) is required for both these activities. The pRN1 primpol primase activity prefers dNTPs to rNTPs; however incorporation of dNTPs requires rNTP as cofactor. This group also includes the Pol domain of bacterial LigD proteins such Mycobacterium tuberculosis (Mt)LigD. MtLigD contains an N-terminal Pol domain, a central phosphoesterase module, and a C-terminal ligase domain. LigD Pol plays a role in non-homologous end joining (NHEJ)-mediated repair of DNA double-strand breaks (DSB) in vivo, perhaps by filling in short 5'-overhangs with ribonucleotides; the filled in termini would be sealed by the associated LigD ligase domain. The MtLigD Pol domain is stimulated by manganese, is error-prone, and prefers adding rNTPs to dNTPs in vitro. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.