| |||||||||||||||||||||||||||||||||||||||||||||
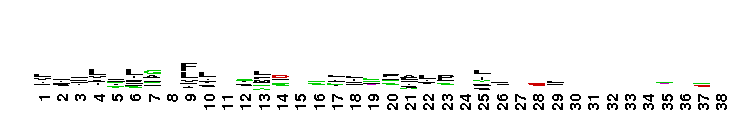
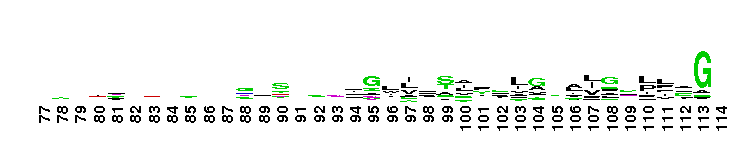
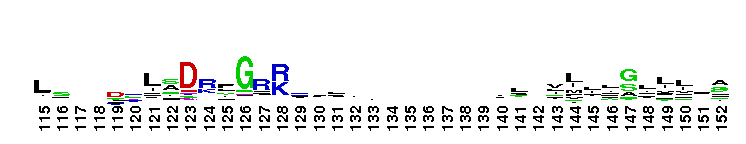
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
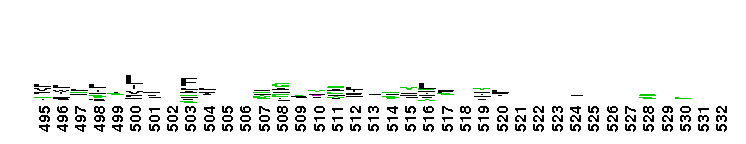
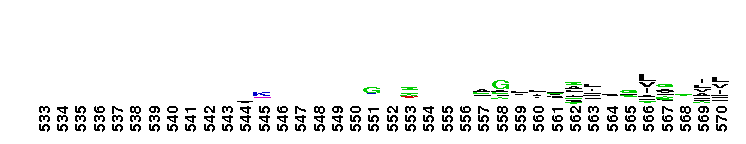
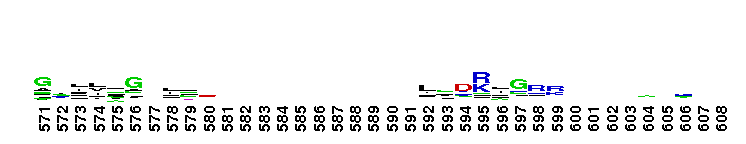
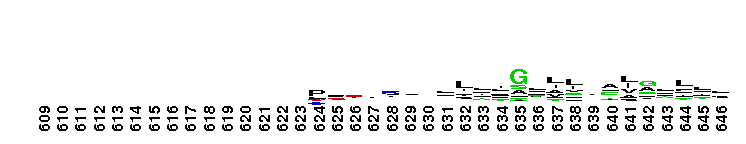
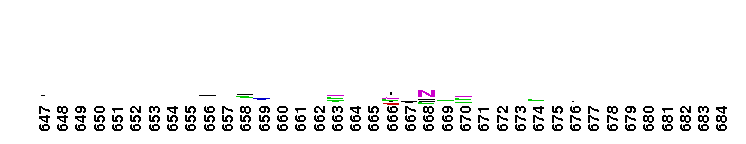
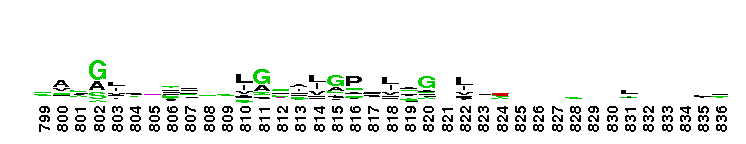
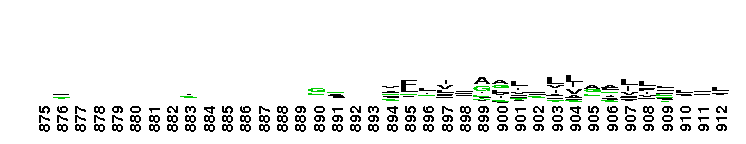
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 The Major Facilitator Superfamily (MFS) is a large and diverse group of secondary transporters that includes uniporters, symporters, and antiporters. MFS proteins facilitate the transport across cytoplasmic or internal membranes of a variety of substrates including ions, sugar phosphates, drugs, neurotransmitters, nucleosides, amino acids, and peptides. They do so using the electrochemical potential of the transported substrates. Uniporters transport a single substrate, while symporters and antiporters transport two substrates in the same or in opposite directions, respectively, across membranes. MFS proteins are typically 400 to 600 amino acids in length, and the majority contain 12 transmembrane alpha helices (TMs) connected by hydrophilic loops. The N- and C-terminal halves of these proteins display weak similarity and may be the result of a gene duplication/fusion event. Based on kinetic studies and the structures of a few bacterial superfamily members, GlpT (glycerol-3-phosphate transporter), LacY (lactose permease), and EmrD (multidrug transporter), MFS proteins are thought to function through a single substrate binding site, alternating-access mechanism involving a rocker-switch type of movement. Bacterial members function primarily for nutrient uptake, and as drug-efflux pumps to confer antibiotic resistance. Some MFS proteins have medical significance in humans such as the glucose transporter Glut4, which is impaired in type II diabetes, and glucose-6-phosphate transporter (G6PT), which causes glycogen storage disease when mutated.
The Major Facilitator Superfamily (MFS) is a large and diverse group of secondary transporters that includes uniporters, symporters, and antiporters. MFS proteins facilitate the transport across cytoplasmic or internal membranes of a variety of substrates including ions, sugar phosphates, drugs, neurotransmitters, nucleosides, amino acids, and peptides. They do so using the electrochemical potential of the transported substrates. Uniporters transport a single substrate, while symporters and antiporters transport two substrates in the same or in opposite directions, respectively, across membranes. MFS proteins are typically 400 to 600 amino acids in length, and the majority contain 12 transmembrane alpha helices (TMs) connected by hydrophilic loops. The N- and C-terminal halves of these proteins display weak similarity and may be the result of a gene duplication/fusion event. Based on kinetic studies and the structures of a few bacterial superfamily members, GlpT (glycerol-3-phosphate transporter), LacY (lactose permease), and EmrD (multidrug transporter), MFS proteins are thought to function through a single substrate binding site, alternating-access mechanism involving a rocker-switch type of movement. Bacterial members function primarily for nutrient uptake, and as drug-efflux pumps to confer antibiotic resistance. Some MFS proteins have medical significance in humans such as the glucose transporter Glut4, which is impaired in type II diabetes, and glucose-6-phosphate transporter (G6PT), which causes glycogen storage disease when mutated. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.