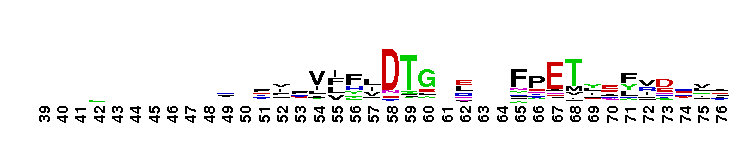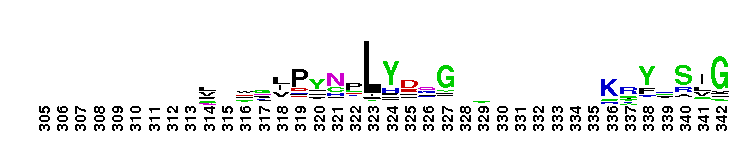| |||||||||||||||||||
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




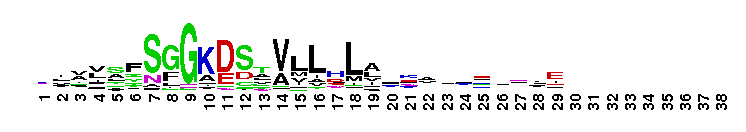
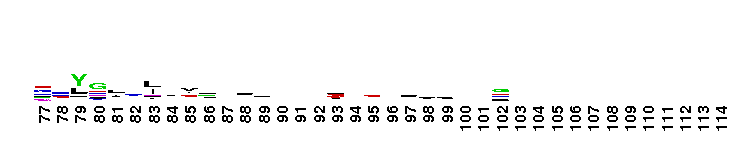
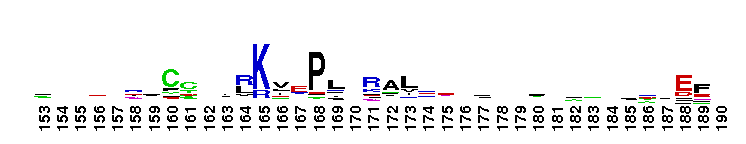
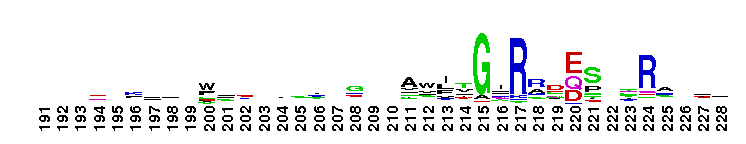
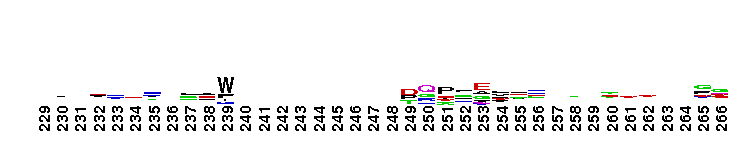
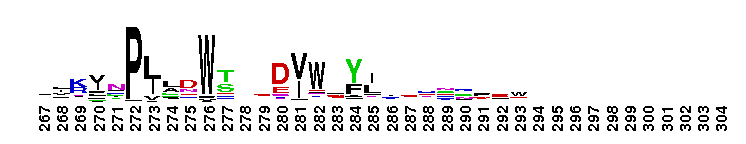
 This domain is found in phosphoadenosine phosphosulphate (PAPS) reductase enzymes or PAPS sulphotransferase. PAPS reductase is part of the adenine nucleotide alpha hydrolases superfamily also including N type ATP PPases and ATP sulphurylases. A highly modified version of the P loop, the fingerprint peptide of mononucleotide-binding proteins, is present in the active site of the protein, which appears to be a positively charged cleft containing a number of conserved arginine and lysine residues. Although PAPS reductase has no ATPase activity, it shows a striking similarity to the structure of the ATP pyrophosphatase (ATP PPase) domain of GMP synthetase, indicating that both enzyme families have evolved from a common ancestral nucleotide-binding fold. The enzyme uses thioredoxin as an electron donor for the reduction of PAPS to phospho-adenosine-phosphate (PAP) . It is also found in NodP nodulation protein P from Rhizobium meliloti which has ATP sulphurylase activity (sulphate adenylate transferase) .
This domain is found in phosphoadenosine phosphosulphate (PAPS) reductase enzymes or PAPS sulphotransferase. PAPS reductase is part of the adenine nucleotide alpha hydrolases superfamily also including N type ATP PPases and ATP sulphurylases. A highly modified version of the P loop, the fingerprint peptide of mononucleotide-binding proteins, is present in the active site of the protein, which appears to be a positively charged cleft containing a number of conserved arginine and lysine residues. Although PAPS reductase has no ATPase activity, it shows a striking similarity to the structure of the ATP pyrophosphatase (ATP PPase) domain of GMP synthetase, indicating that both enzyme families have evolved from a common ancestral nucleotide-binding fold. The enzyme uses thioredoxin as an electron donor for the reduction of PAPS to phospho-adenosine-phosphate (PAP) . It is also found in NodP nodulation protein P from Rhizobium meliloti which has ATP sulphurylase activity (sulphate adenylate transferase) . No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.