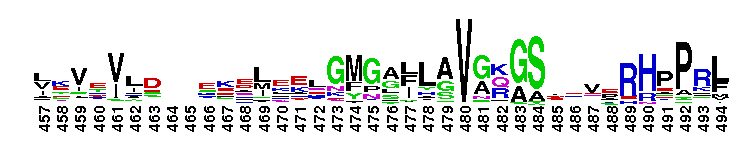| |||||||||||||||||||
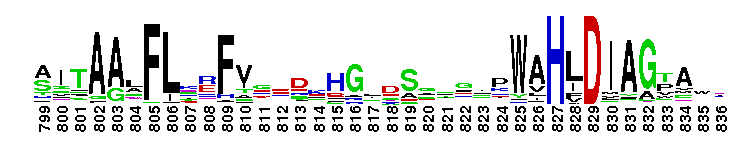
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 Cytosol aminopeptidase family, N-terminal and catalytic domains. Family M17 contains zinc- and manganese-dependent exopeptidases ( EC 3.4.11.1), including leucine aminopeptidase. They catalyze removal of amino acids from the N-terminus of a protein and play a key role in protein degradation and in the metabolism of biologically active peptides. They do not contain HEXXH motif (which is used as one of the signature patterns to group the peptidase families) in the metal-binding site. The two associated zinc ions and the active site are entirely enclosed within the C-terminal catalytic domain in leucine aminopeptidase. The enzyme is a hexamer, with the catalytic domains clustered around the three-fold axis, and the two trimers related to one another by a two-fold rotation. The N-terminal domain is structurally similar to the ADP-ribose binding Macro domain. This family includes proteins from bacteria, archaea, animals and plants.
Cytosol aminopeptidase family, N-terminal and catalytic domains. Family M17 contains zinc- and manganese-dependent exopeptidases ( EC 3.4.11.1), including leucine aminopeptidase. They catalyze removal of amino acids from the N-terminus of a protein and play a key role in protein degradation and in the metabolism of biologically active peptides. They do not contain HEXXH motif (which is used as one of the signature patterns to group the peptidase families) in the metal-binding site. The two associated zinc ions and the active site are entirely enclosed within the C-terminal catalytic domain in leucine aminopeptidase. The enzyme is a hexamer, with the catalytic domains clustered around the three-fold axis, and the two trimers related to one another by a two-fold rotation. The N-terminal domain is structurally similar to the ADP-ribose binding Macro domain. This family includes proteins from bacteria, archaea, animals and plants. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.