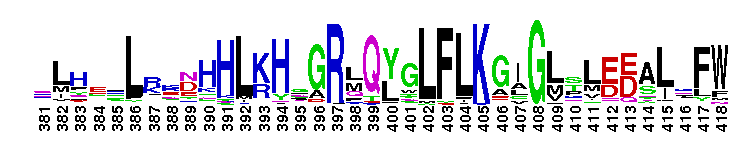| |||||||||||||||||||
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




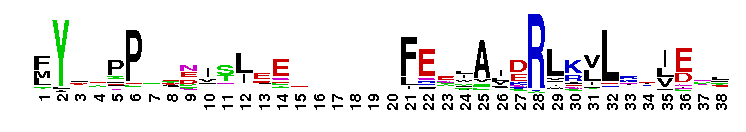
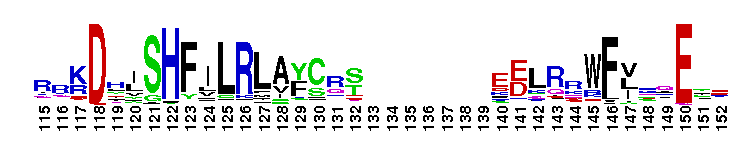
 Eukaryotic core primase: Large subunit, PriL. Primases synthesize the RNA primers required for DNA replication. Primases are grouped into two classes, bacteria/bacteriophage and archaeal/eukaryotic. The proteins in the two classes differ in structure and the replication apparatus components. Archaeal/eukaryotic core primase is a heterodimeric enzyme consisting of a small catalytic subunit (PriS) and a large subunit (PriL). In eukaryotic organisms, a heterotetrameric enzyme formed by DNA polymerase alpha, the B subunit and two primase subunits has primase activity. Although the catalytic activity resides within PriS, the PriL subunit is essential for primase function as disruption of the PriL gene in yeast is lethal. PriL is composed of two structural domains. Several functions have been proposed for PriL such as stabilization of the PriS, involvement in synthesis initiation, improvement of primase processivity, determination of product size and transfer of the products to DNA polymerase alpha.
Eukaryotic core primase: Large subunit, PriL. Primases synthesize the RNA primers required for DNA replication. Primases are grouped into two classes, bacteria/bacteriophage and archaeal/eukaryotic. The proteins in the two classes differ in structure and the replication apparatus components. Archaeal/eukaryotic core primase is a heterodimeric enzyme consisting of a small catalytic subunit (PriS) and a large subunit (PriL). In eukaryotic organisms, a heterotetrameric enzyme formed by DNA polymerase alpha, the B subunit and two primase subunits has primase activity. Although the catalytic activity resides within PriS, the PriL subunit is essential for primase function as disruption of the PriL gene in yeast is lethal. PriL is composed of two structural domains. Several functions have been proposed for PriL such as stabilization of the PriS, involvement in synthesis initiation, improvement of primase processivity, determination of product size and transfer of the products to DNA polymerase alpha. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.