| |||||||||||||||||||
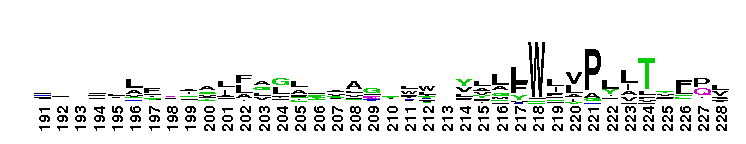
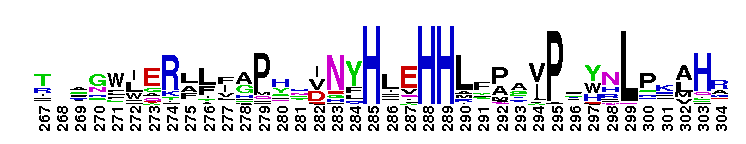
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




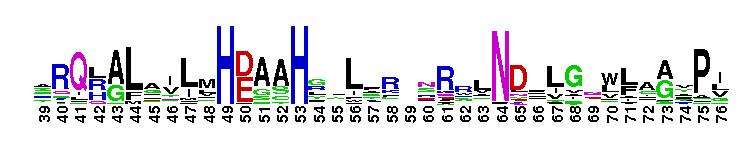
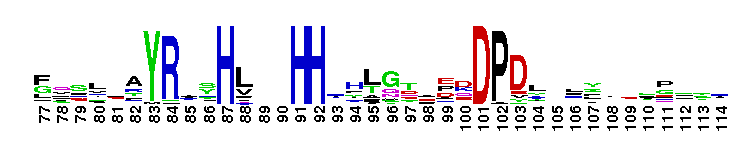
 This CD includes the dihydrorhizobitoxine fatty acid desaturase (RtxC) characterized in Bradyrhizobium japonicum USDA110, and other related proteins. Dihydrorhizobitoxine desaturase is reported to be involved in the final step of rhizobitoxine biosynthesis. This domain family appears to be structurally related to the membrane fatty acid desaturases and the alkane hydroxylases. They all share in common extensive hydrophobic regions that would be capable of spanning the membrane bilayer at least twice. Comparison of sequences also reveals the existence of three regions of conserved histidine cluster motifs that contain eight histidine residues: HXXXH, HXX(X)HH, and HXXHH. These histidine residues are reported to be catalytically essential and proposed to be the ligands for the iron atoms contained within homologs, stearoyl CoA desaturase and alkane hydroxylase.
This CD includes the dihydrorhizobitoxine fatty acid desaturase (RtxC) characterized in Bradyrhizobium japonicum USDA110, and other related proteins. Dihydrorhizobitoxine desaturase is reported to be involved in the final step of rhizobitoxine biosynthesis. This domain family appears to be structurally related to the membrane fatty acid desaturases and the alkane hydroxylases. They all share in common extensive hydrophobic regions that would be capable of spanning the membrane bilayer at least twice. Comparison of sequences also reveals the existence of three regions of conserved histidine cluster motifs that contain eight histidine residues: HXXXH, HXX(X)HH, and HXXHH. These histidine residues are reported to be catalytically essential and proposed to be the ligands for the iron atoms contained within homologs, stearoyl CoA desaturase and alkane hydroxylase. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.