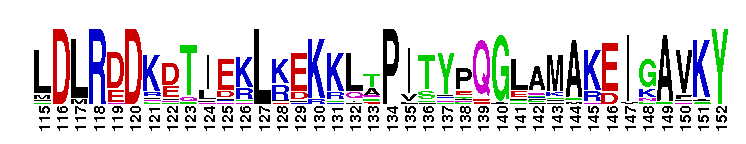| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 Ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1)-like consists of Rac1, Rac2 and Rac3. The Rac1-like subfamily consists of Rac1, Rac2, and Rac3 proteins, plus the splice variant Rac1b that contains a 19-residue insertion near switch II relative to Rac1. While Rac1 is ubiquitously expressed, Rac2 and Rac3 are largely restricted to hematopoietic and neural tissues respectively. Rac1 stimulates the formation of actin lamellipodia and membrane ruffles. It also plays a role in cell-matrix adhesion and cell anoikis. In intestinal epithelial cells, Rac1 is an important regulator of migration and mediates apoptosis. Rac1 is also essential for RhoA-regulated actin stress fiber and focal adhesion complex formation. In leukocytes, Rac1 and Rac2 have distinct roles in regulating cell morphology, migration, and invasion, but are not essential for macrophage migration or chemotaxis. Rac3 has biochemical properties that are closely related to Rac1, such as effector interaction, nucleotide binding, and hydrolysis; Rac2 has a slower nucleotide association and is more efficiently activated by the RacGEF Tiam1. Both Rac1 and Rac3 have been implicated in the regulation of cell migration and invasion in human metastatic breast cancer. Most Rho proteins contain a lipid modification site at the C-terminus, with a typical sequence motif CaaX, where a = an aliphatic amino acid and X = any amino acid. Lipid binding is essential for membrane attachment, a key feature of most Rho proteins. Due to the presence of truncated sequences in this CD, the lipid modification site is not available for annotation.
Ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1)-like consists of Rac1, Rac2 and Rac3. The Rac1-like subfamily consists of Rac1, Rac2, and Rac3 proteins, plus the splice variant Rac1b that contains a 19-residue insertion near switch II relative to Rac1. While Rac1 is ubiquitously expressed, Rac2 and Rac3 are largely restricted to hematopoietic and neural tissues respectively. Rac1 stimulates the formation of actin lamellipodia and membrane ruffles. It also plays a role in cell-matrix adhesion and cell anoikis. In intestinal epithelial cells, Rac1 is an important regulator of migration and mediates apoptosis. Rac1 is also essential for RhoA-regulated actin stress fiber and focal adhesion complex formation. In leukocytes, Rac1 and Rac2 have distinct roles in regulating cell morphology, migration, and invasion, but are not essential for macrophage migration or chemotaxis. Rac3 has biochemical properties that are closely related to Rac1, such as effector interaction, nucleotide binding, and hydrolysis; Rac2 has a slower nucleotide association and is more efficiently activated by the RacGEF Tiam1. Both Rac1 and Rac3 have been implicated in the regulation of cell migration and invasion in human metastatic breast cancer. Most Rho proteins contain a lipid modification site at the C-terminus, with a typical sequence motif CaaX, where a = an aliphatic amino acid and X = any amino acid. Lipid binding is essential for membrane attachment, a key feature of most Rho proteins. Due to the presence of truncated sequences in this CD, the lipid modification site is not available for annotation. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.