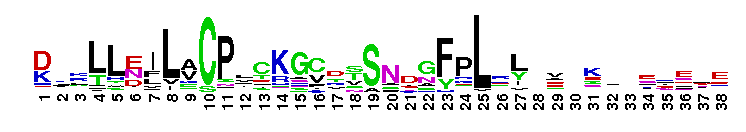| |||||||||||||||||||
Tips:  Range on the Protein: Protein ID Protein Position Domain Position:  No Conserved Features/Sites Found for Trm112p No Conserved Features/Sites Found for Trm112p
|
|---|
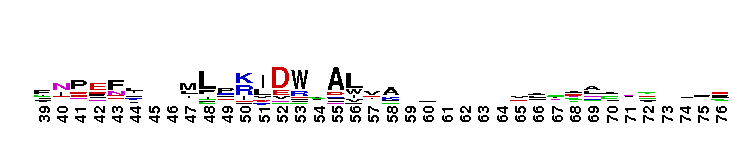
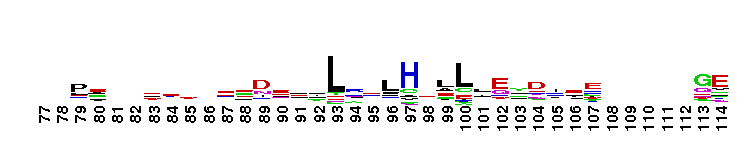
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




 Trm112p-like protein. The function of this family is uncertain. The bacterial members are about 60-70 amino acids in length and the eukaryotic examples are about 120 amino acids in length. The C terminus contains the strongest conservation. Trm112p is required for tRNA methylation in S. cerevisiae and is found in complexes with 2 tRNA methylases (TRM9 and TRM11) also with putative methyltransferase YDR140W. The zinc-finger protein Ynr046w is plurifunctional and a component of the eRF1 methyltransferase in yeast. The crystal structure of Ynr046w has been determined to 1.7 A resolution. It comprises a zinc-binding domain built from both the N- and C-terminal sequences and an inserted domain, absent from bacterial and archaeal orthologs of the protein, composed of three alpha-helices.
Trm112p-like protein. The function of this family is uncertain. The bacterial members are about 60-70 amino acids in length and the eukaryotic examples are about 120 amino acids in length. The C terminus contains the strongest conservation. Trm112p is required for tRNA methylation in S. cerevisiae and is found in complexes with 2 tRNA methylases (TRM9 and TRM11) also with putative methyltransferase YDR140W. The zinc-finger protein Ynr046w is plurifunctional and a component of the eRF1 methyltransferase in yeast. The crystal structure of Ynr046w has been determined to 1.7 A resolution. It comprises a zinc-binding domain built from both the N- and C-terminal sequences and an inserted domain, absent from bacterial and archaeal orthologs of the protein, composed of three alpha-helices. No pairwise interactions found for the domain Trm112p
No pairwise interactions found for the domain Trm112p