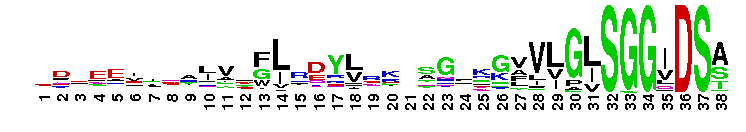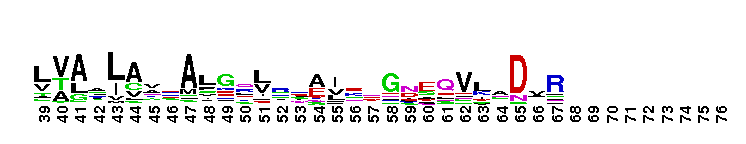| |||||||||||||||||||
Tips:  Range on the Protein: Protein ID Protein Position Domain Position: 
|
|---|
Weblogos are Copyright (c) 2002 Regents of the University of California
| DMDM_info@umbc.edu | 1000 Hilltop Circle, Baltimore, MD 21250 | Department of Biological Sciences | Phone: 410-455-2258 |




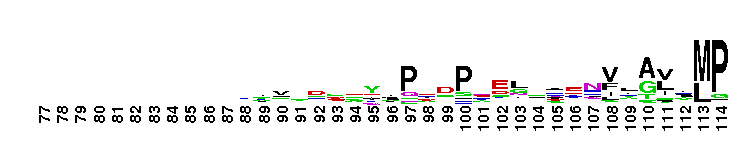
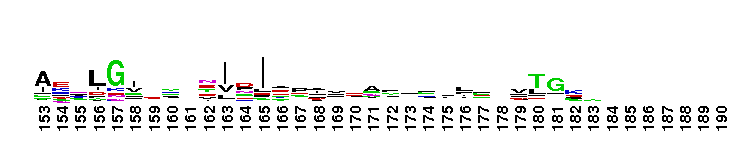
 NAD+ synthase is a homodimer, which catalyzes the final step in de novo nicotinamide adenine dinucleotide (NAD+) biosynthesis, an amide transfer from either ammonia or glutamine to nicotinic acid adenine dinucleotide (NaAD). The conversion of NaAD to NAD+ occurs via an NAD-adenylate intermediate and requires ATP and Mg2+. The intemediate is subsequently cleaved into NAD+ and AMP. In many prokaryotes, such as E. coli , NAD synthetase consists of a single domain and is strictly ammonia dependent. In contrast, eukaryotes and other prokaryotes have an additional N-terminal amidohydrolase domain that prefer glutamine, Interestingly, NAD+ synthases in these prokaryotes, can also utilize ammonia as an amide source .
NAD+ synthase is a homodimer, which catalyzes the final step in de novo nicotinamide adenine dinucleotide (NAD+) biosynthesis, an amide transfer from either ammonia or glutamine to nicotinic acid adenine dinucleotide (NaAD). The conversion of NaAD to NAD+ occurs via an NAD-adenylate intermediate and requires ATP and Mg2+. The intemediate is subsequently cleaved into NAD+ and AMP. In many prokaryotes, such as E. coli , NAD synthetase consists of a single domain and is strictly ammonia dependent. In contrast, eukaryotes and other prokaryotes have an additional N-terminal amidohydrolase domain that prefer glutamine, Interestingly, NAD+ synthases in these prokaryotes, can also utilize ammonia as an amide source . No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.