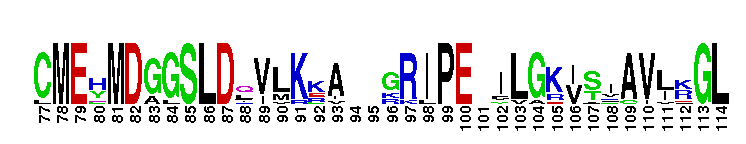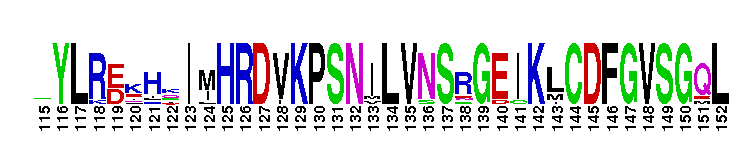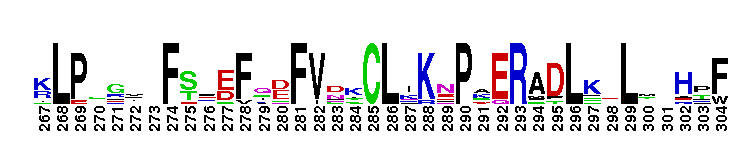ADENOCARCINOMA OF LUNG, RESPONSE TO TYROS ADENOCARCINOMA OF LUNG, RESPONSE TO TYROS
|
 ADENOCARCINOMA OF LUNG, SOMATIC ADENOCARCINOMA OF LUNG, SOMATIC
|
 AMYOTROPHIC LATERAL SCLEROSIS 19 AMYOTROPHIC LATERAL SCLEROSIS 19
|
 AORTIC ANEURYSM, FAMILIAL THORACIC 7 AORTIC ANEURYSM, FAMILIAL THORACIC 7
|
 BLADDER CANCER, SOMATIC, INCLUDED BLADDER CANCER, SOMATIC, INCLUDED
|
 BRACHYDACTYLY, TYPE A2 BRACHYDACTYLY, TYPE A2
|
 BREAST CANCER, SUSCEPTIBILITY TO BREAST CANCER, SUSCEPTIBILITY TO
|
 CAMPTODACTYLY, TALL STATURE, AND HEARING LOSS SYNDROME CAMPTODACTYLY, TALL STATURE, AND HEARING LOSS SYNDROME
|
 CARDIOFACIOCUTANEOUS SYNDROME 1 CARDIOFACIOCUTANEOUS SYNDROME 1
|
 CARDIOFACIOCUTANEOUS SYNDROME 3 CARDIOFACIOCUTANEOUS SYNDROME 3
|
 CARDIOFACIOCUTANEOUS SYNDROME 4 CARDIOFACIOCUTANEOUS SYNDROME 4
|
 CATARACT 6, AGE-RELATED CORTICAL CATARACT 6, AGE-RELATED CORTICAL
|
 CHRONIC MYELOID LEUKEMIA, RESISTANT TO IMATINIB CHRONIC MYELOID LEUKEMIA, RESISTANT TO IMATINIB
|
 COFFIN-LOWRY SYNDROME COFFIN-LOWRY SYNDROME
|
 COFFIN-LOWRY SYNDROME, MILD COFFIN-LOWRY SYNDROME, MILD
|
 COLON CANCER, HEREDITARY NONPOLYPOSIS, TYPE 6, SOMATIC, INCLUDED COLON CANCER, HEREDITARY NONPOLYPOSIS, TYPE 6, SOMATIC, INCLUDED
|
 COLON CANCER, SOMATIC COLON CANCER, SOMATIC
|
 COLORECTAL CANCER, SOMATIC COLORECTAL CANCER, SOMATIC
|
 COLORECTAL CANCER, SOMATIC, INCLUDED;; COLORECTAL CANCER, SOMATIC, INCLUDED;;
|
 CROUZON SYNDROME CROUZON SYNDROME
|
 DEFICIENCY DEFICIENCY
|
 DIABETES MELLITUS, INSULIN-RESISTANT, WITH ACANTHOSIS NIGRICANS DIABETES MELLITUS, INSULIN-RESISTANT, WITH ACANTHOSIS NIGRICANS
|
 DIABETES MELLITUS, NONINSULIN-DEPENDENT DIABETES MELLITUS, NONINSULIN-DEPENDENT
|
 DIABETES MELLITUS, TYPE II DIABETES MELLITUS, TYPE II
|
 ENDOCRINE-CEREBROOSTEODYSPLASIA ENDOCRINE-CEREBROOSTEODYSPLASIA
|
 EPILEPTIC ENCEPHALOPATHY, EARLY INFANTILE, 2 EPILEPTIC ENCEPHALOPATHY, EARLY INFANTILE, 2
|
 ESOPHAGEAL CANCER, SOMATIC ESOPHAGEAL CANCER, SOMATIC
|
 FG SYNDROME 4 FG SYNDROME 4
|
 FIBRODYSPLASIA OSSIFICANS PROGRESSIVA FIBRODYSPLASIA OSSIFICANS PROGRESSIVA
|
 GASTRIC CANCER, SOMATIC GASTRIC CANCER, SOMATIC
|
 GLIOBLASTOMA, SOMATIC GLIOBLASTOMA, SOMATIC
|
 GLYCOGEN STORAGE DISEASE IXC GLYCOGEN STORAGE DISEASE IXC
|
 HARTSFIELD SYNDROME HARTSFIELD SYNDROME
|
 HIRSCHSPRUNG DISEASE, SUSCEPTIBILITY TO, 1 HIRSCHSPRUNG DISEASE, SUSCEPTIBILITY TO, 1
|
 HYPERINSULINEMIC HYPOGLYCEMIA, FAMILIAL, 5 HYPERINSULINEMIC HYPOGLYCEMIA, FAMILIAL, 5
|
 HYPOCHONDROPLASIA HYPOCHONDROPLASIA
|
 HYPOGONADOTROPIC HYPOGONADISM 2 WITH ANOSMIA HYPOGONADOTROPIC HYPOGONADISM 2 WITH ANOSMIA
|
 HYPOGONADOTROPIC HYPOGONADISM 2 WITH ANOSMIA, SUSCEPTIBILITY TO HYPOGONADOTROPIC HYPOGONADISM 2 WITH ANOSMIA, SUSCEPTIBILITY TO
|
 HYPOGONADOTROPIC HYPOGONADISM 2 WITH OR WITHOUT ANOSMIA, SUSCEPTIBILITY HYPOGONADOTROPIC HYPOGONADISM 2 WITH OR WITHOUT ANOSMIA, SUSCEPTIBILITY
|
 HYPOGONADOTROPIC HYPOGONADISM 2 WITHOUT ANOSMIA HYPOGONADOTROPIC HYPOGONADISM 2 WITHOUT ANOSMIA
|
 HYPOGONADOTROPIC HYPOGONADISM 2 WITHOUT ANOSMIA, SUSCEPTIBILITY TO HYPOGONADOTROPIC HYPOGONADISM 2 WITHOUT ANOSMIA, SUSCEPTIBILITY TO
|
 IN IN
|
 INSENSITIVITY TO PAIN, CONGENITAL, WITH ANHIDROSIS INSENSITIVITY TO PAIN, CONGENITAL, WITH ANHIDROSIS
|
 INSULIN RESISTANCE INSULIN RESISTANCE
|
 INSULIN RESISTANCE, INCLUDED INSULIN RESISTANCE, INCLUDED
|
 IRAK4 DEFICIENCY IRAK4 DEFICIENCY
|
 JUVENILE POLYPOSIS SYNDROME JUVENILE POLYPOSIS SYNDROME
|
 LADD SYNDROME LADD SYNDROME
|
 LEUKEMIA, PHILADELPHIA CHROMOSOME-POSITIVE, RESISTANT TO IMATINIB LEUKEMIA, PHILADELPHIA CHROMOSOME-POSITIVE, RESISTANT TO IMATINIB
|
 LOEYS-DIETZ SYNDROME, TYPE 1B LOEYS-DIETZ SYNDROME, TYPE 1B
|
 LOEYS-DIETZ SYNDROME, TYPE 2B LOEYS-DIETZ SYNDROME, TYPE 2B
|
 LYMPHOMA, NON-HODGKIN, SOMATIC LYMPHOMA, NON-HODGKIN, SOMATIC
|
 LYMPHOPROLIFERATIVE SYNDROME 1 LYMPHOPROLIFERATIVE SYNDROME 1
|
 MALFORMATIONS MALFORMATIONS
|
 MEGALENCEPHALY-POLYMICROGYRIA-POLYDACTYLY-HYDROCEPHALUS SYNDROME MEGALENCEPHALY-POLYMICROGYRIA-POLYDACTYLY-HYDROCEPHALUS SYNDROME
|
 MELANOMA, MALIGNANT, SOMATIC MELANOMA, MALIGNANT, SOMATIC
|
 MENTAL RETARDATION AND MICROCEPHALY WITH PONTINE AND CEREBELLAR HYPOPLASIA MENTAL RETARDATION AND MICROCEPHALY WITH PONTINE AND CEREBELLAR HYPOPLASIA
|
 MENTAL RETARDATION, X-LINKED 19 MENTAL RETARDATION, X-LINKED 19
|
 MENTAL RETARDATION, X-LINKED 30 MENTAL RETARDATION, X-LINKED 30
|
 MENTAL RETARDATION, X-LINKED, WITH NYSTAGMUS MENTAL RETARDATION, X-LINKED, WITH NYSTAGMUS
|
 MULTIPLE ENDOCRINE NEOPLASIA, TYPE IIA MULTIPLE ENDOCRINE NEOPLASIA, TYPE IIA
|
 MULTIPLE ENDOCRINE NEOPLASIA, TYPE IIB MULTIPLE ENDOCRINE NEOPLASIA, TYPE IIB
|
 MULTIPLE MYELOMA, SOMATIC, INCLUDED;; MULTIPLE MYELOMA, SOMATIC, INCLUDED;;
|
 MYASTHENIC SYNDROME, CONGENITAL, ASSOCIATED WITH ACETYLCHOLINE RECEPTOR MYASTHENIC SYNDROME, CONGENITAL, ASSOCIATED WITH ACETYLCHOLINE RECEPTOR
|
 NEPHRONOPHTHISIS 9 (NPHP9) NEPHRONOPHTHISIS 9 (NPHP9)
|
 NEUROPATHY, HEREDITARY SENSORY, TYPE II NEUROPATHY, HEREDITARY SENSORY, TYPE II
|
 NONSMALL CELL LUNG CANCER, RESISTANCE TO TYROSINE KINASE INHIBITOR NONSMALL CELL LUNG CANCER, RESISTANCE TO TYROSINE KINASE INHIBITOR
|
 NONSMALL CELL LUNG CANCER, RESPONSE TO TYROSINE KINASE INHIBITOR IN, NONSMALL CELL LUNG CANCER, RESPONSE TO TYROSINE KINASE INHIBITOR IN,
|
 NOONAN SYNDROME 7 NOONAN SYNDROME 7
|
 OBESITY, HYPERPHAGIA, AND DEVELOPMENTAL DELAY OBESITY, HYPERPHAGIA, AND DEVELOPMENTAL DELAY
|
 OGUCHI DISEASE 2 OGUCHI DISEASE 2
|
 OVARIAN CANCER, SOMATIC OVARIAN CANCER, SOMATIC
|
 PARKINSON DISEASE 8, AUTOSOMAL DOMINANT PARKINSON DISEASE 8, AUTOSOMAL DOMINANT
|
 PEUTZ-JEGHERS SYNDROME PEUTZ-JEGHERS SYNDROME
|
 PFEIFFER SYNDROME PFEIFFER SYNDROME
|
 PHEOCHROMOCYTOMA, INCLUDED PHEOCHROMOCYTOMA, INCLUDED
|
 PHEOCHROMOCYTOMA, SOMATIC, IN PHEOCHROMOCYTOMA, SOMATIC, IN
|
 PROSTATE CANCER, PROGRESSION AND METASTASIS OF PROSTATE CANCER, PROGRESSION AND METASTASIS OF
|
 PULMONARY HYPERTENSION, PRIMARY, 1 PULMONARY HYPERTENSION, PRIMARY, 1
|
 PULMONARY HYPERTENSION, PRIMARY, 1, WITH HEREDITARY HEMORRHAGIC TELANGIECTASIA PULMONARY HYPERTENSION, PRIMARY, 1, WITH HEREDITARY HEMORRHAGIC TELANGIECTASIA
|
 PULMONARY HYPERTENSION, PRIMARY, DEXFENFLURAMINE-ASSOCIATED, INCLUDED PULMONARY HYPERTENSION, PRIMARY, DEXFENFLURAMINE-ASSOCIATED, INCLUDED
|
 PULMONARY VENOOCCLUSIVE DISEASE PULMONARY VENOOCCLUSIVE DISEASE
|
 RENAL AGENESIS RENAL AGENESIS
|
 RETINITIS PIGMENTOSA 62 RETINITIS PIGMENTOSA 62
|
 RETINITIS PIGMENTOSA 62 (RP62) RETINITIS PIGMENTOSA 62 (RP62)
|
 SADDAN DYSPLASIA SADDAN DYSPLASIA
|
 SCAPHOCEPHALY, MAXILLARY RETRUSION, AND MENTAL RETARDATION, INCLUDED SCAPHOCEPHALY, MAXILLARY RETRUSION, AND MENTAL RETARDATION, INCLUDED
|
 SELECTIVE T-CELL DEFECT SELECTIVE T-CELL DEFECT
|
 SHORT RIB-POLYDACTYLY SYNDROME 2A (SRPS2A) SHORT RIB-POLYDACTYLY SYNDROME 2A (SRPS2A)
|
 SHORT RIB-POLYDACTYLY SYNDROME, TYPE IIA SHORT RIB-POLYDACTYLY SYNDROME, TYPE IIA
|
 SOMATIC SOMATIC
|
 SPERMATOCYTIC SEMINOMA, SOMATIC, INCLUDED SPERMATOCYTIC SEMINOMA, SOMATIC, INCLUDED
|
 SPERMATOGENIC FAILURE 5 SPERMATOGENIC FAILURE 5
|
 SPINOCEREBELLAR ATAXIA 14 SPINOCEREBELLAR ATAXIA 14
|
 SPONDYLOMETAEPIPHYSEAL DYSPLASIA, SHORT LIMB-HAND TYPE SPONDYLOMETAEPIPHYSEAL DYSPLASIA, SHORT LIMB-HAND TYPE
|
 T-CELL IMMUNODEFICIENCY, RECURRENT INFECTIONS, AUTOIMMUNITY, AND CARDIAC T-CELL IMMUNODEFICIENCY, RECURRENT INFECTIONS, AUTOIMMUNITY, AND CARDIAC
|
 TESTICULAR TUMOR, SOMATIC TESTICULAR TUMOR, SOMATIC
|
 THANATOPHORIC DYSPLASIA, TYPE I THANATOPHORIC DYSPLASIA, TYPE I
|
 THANATOPHORIC DYSPLASIA, TYPE I, INCLUDED THANATOPHORIC DYSPLASIA, TYPE I, INCLUDED
|
 THANATOPHORIC DYSPLASIA, TYPE II THANATOPHORIC DYSPLASIA, TYPE II
|
 THROMBOCYTOPENIA 2 THROMBOCYTOPENIA 2
|
 THYROID CARCINOMA, FAMILIAL MEDULLARY THYROID CARCINOMA, FAMILIAL MEDULLARY
|
 THYROID CARCINOMA, FAMILIAL MEDULLARY, INCLUDED THYROID CARCINOMA, FAMILIAL MEDULLARY, INCLUDED
|
 THYROID CARCINOMA, FOLLICULAR, SOMATIC, INCLUDED THYROID CARCINOMA, FOLLICULAR, SOMATIC, INCLUDED
|
 THYROID CARCINOMA, PAPILLARY, SOMATIC, INCLUDED;;| THYROID CARCINOMA, PAPILLARY, SOMATIC, INCLUDED;;|
|
 THYROID CARCINOMA, SPORADIC MEDULLARY, INCLUDED;; THYROID CARCINOMA, SPORADIC MEDULLARY, INCLUDED;;
|
 TO TO
|
 VENOUS MALFORMATIONS, MULTIPLE CUTANEOUS AND MUCOSAL VENOUS MALFORMATIONS, MULTIPLE CUTANEOUS AND MUCOSAL
|






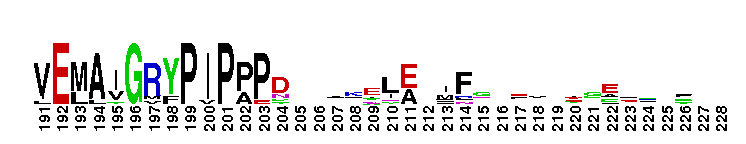
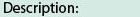 Catalytic domain of the dual-specificity Protein Kinase, MAP/ERK Kinase. Protein kinases (PKs), MAP/ERK kinase (MEK) subfamily, catalytic (c) domain. PKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine or tyrosine residues on protein substrates. The MEK subfamily is part of a larger superfamily that includes the catalytic domains of other protein serine/threonine kinases, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. The mitogen-activated protein (MAP) kinase signaling pathways are important mediators of cellular responses to extracellular signals. The pathways involve a triple kinase core cascade comprising the MAP kinase (MAPK), which is phosphorylated and activated by a MAPK kinase (MAPKK or MKK), which itself is phosphorylated and activated by a MAPK kinase kinase (MAPKKK or MKKK). MEK1 and MEK2 are dual-specificity PKs that phosphorylate and activate the downstream targets, ERK(extracellular signal-regulated kinase) 1 and ERK2, on specific threonine and tyrosine residues. The ERK cascade starts with extracellular signals including growth factors, hormones, and neurotransmitters, which act through receptors and ion channels to initiate intracellular signaling that leads to the activation at the MAPKKK (Raf-1 or MOS) level, which leads to the transmission of signals to MEK1/2, and finally to ERK1/2. The ERK cascade plays an important role in cell proliferation, differentiation, oncogenic transformation, and cell cycle control, as well as in apoptosis and cell survival under certain conditions. This cascade has also been implicated in synaptic plasticity, migration, morphological determination, and stress response immunological reactions. Gain-of-function mutations in genes encoding ERK cascade proteins, including MEK1/2, cause cardiofaciocutaneous (CFC) syndrome, a condition leading to multiple congenital anomalies and mental retardation in patients.
Catalytic domain of the dual-specificity Protein Kinase, MAP/ERK Kinase. Protein kinases (PKs), MAP/ERK kinase (MEK) subfamily, catalytic (c) domain. PKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine or tyrosine residues on protein substrates. The MEK subfamily is part of a larger superfamily that includes the catalytic domains of other protein serine/threonine kinases, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. The mitogen-activated protein (MAP) kinase signaling pathways are important mediators of cellular responses to extracellular signals. The pathways involve a triple kinase core cascade comprising the MAP kinase (MAPK), which is phosphorylated and activated by a MAPK kinase (MAPKK or MKK), which itself is phosphorylated and activated by a MAPK kinase kinase (MAPKKK or MKKK). MEK1 and MEK2 are dual-specificity PKs that phosphorylate and activate the downstream targets, ERK(extracellular signal-regulated kinase) 1 and ERK2, on specific threonine and tyrosine residues. The ERK cascade starts with extracellular signals including growth factors, hormones, and neurotransmitters, which act through receptors and ion channels to initiate intracellular signaling that leads to the activation at the MAPKKK (Raf-1 or MOS) level, which leads to the transmission of signals to MEK1/2, and finally to ERK1/2. The ERK cascade plays an important role in cell proliferation, differentiation, oncogenic transformation, and cell cycle control, as well as in apoptosis and cell survival under certain conditions. This cascade has also been implicated in synaptic plasticity, migration, morphological determination, and stress response immunological reactions. Gain-of-function mutations in genes encoding ERK cascade proteins, including MEK1/2, cause cardiofaciocutaneous (CFC) syndrome, a condition leading to multiple congenital anomalies and mental retardation in patients. No pairwise interactions are available for this conserved domain.
No pairwise interactions are available for this conserved domain.